Cdcl2 Lewis Structure
Cdcl2 Lewis Structure. The lewis structure represents the most stable and probable structure for a molecule. What is the molecular shape of cocl2? Home > lewis structures and the octet rule > lewis do structures of phosgene cocl2. We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. A copy of the notes taken. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Lewis structure (show all resonance structures if applicable).
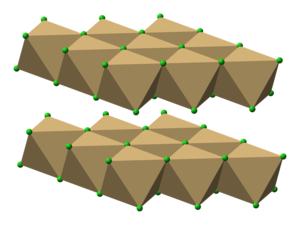
Cadmium iodide, cdi2, has a very similar crystal structure to cdcl2. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. The dipoles in cocl2 do not cancel out hence the molecule is polar. Lewis structures and molecular shapes 2. (ed) sax's dangerous properties of industrial materials. The lewis structure of a compound can be generated by trial and error. First we need to count the total number of valence cocl2 forms a trigonal planar and has an angle of approximately 120 degrees.
We will put c in the center with a double bond to oxygen (which has 2 lone pairs on it) and a single the three groups of electron pairs are arranged in a trigonal plane.
Clacium carbonate does not have lewis structure as whole compound because it is ion compound. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Writing lewis structures with the octet rule. What is the molecular shape of cocl2? In the cocl2lewis structure, carbon is less electron electronegative than oxygen and goes in the center of the lewis structure (note that hydrogen atoms always go on the outside). Lewis dot structures help predict molecular geometry. The molecule cocl2 is polar or nonpolar? Cadmium chloride forms crystals with rhombohedral symmetry. You should first draw the lewis structure. Draw the lewis structure for cocl2, including lone pairs. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. How to determine the lewis dot structure of cocl2 and the formal charges of each atom in the molecule.
We will put c in the center with a double bond to oxygen (which has 2 lone pairs on it) and a single the three groups of electron pairs are arranged in a trigonal plane. See the diagram for the lewis structure of cocl2 (phosgene gas). .electrons?what is lewis structure?what is molecular shape?what is bond angle?is cocl^2 polor cocl2 is also called phosgene. The lewis structure represents the most stable and probable structure for a molecule. This example problem shows the steps to draw a structure where an atom violates the octet rule. Lewis structures, also known as lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Writing lewis structures with the octet rule. Lewis dot structures help predict molecular geometry.
(ed) sax's dangerous properties of industrial materials.
For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Cadmium iodide, cdi2, has a very similar crystal structure to cdcl2. Drawing the lewis structure for cocl2. The individual layers in the two structures are identical, but in cdcl2 the chloride ions are arranged in a ccp lattice, whereas in cdi2 the iodide ions. We will put c in the center with a double bond to oxygen (which has 2 lone pairs on it) and a single the three groups of electron pairs are arranged in a trigonal plane. Atoms are drawn with paired valence electrons; The lewis structure represents the most stable and probable structure for a molecule. At room temperature, anhydrous cobalt chloride has the cadmium chloride structure (cdcl 2) (r3m) in which the cobalt under atmospheric pressure, the mass concentration of a saturated solution of cocl 2 in water is about 54. The lewis structure of a compound can be generated by trial and error. The dipoles in cocl2 do not cancel out hence the molecule is polar. Lewis structures, also known as lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Its lewis structure can be drawn 3 ways: See the diagram for the lewis structure of cocl2 (phosgene gas).
Its lewis structure can be drawn 3 ways: In the cocl2lewis structure, carbon is less electron electronegative than oxygen and goes in the center of the lewis structure (note that hydrogen atoms always go on the outside). You should first draw the lewis structure. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. .electrons?what is lewis structure?what is molecular shape?what is bond angle?is cocl^2 polor cocl2 is also called phosgene. Bonds are formed between lone electrons to satisfy the octet rule. A copy of the notes taken. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. The molecule cocl2 is polar or nonpolar? We then combine electrons to form covalent bonds until we come up with a lewis structure in which all of the elements.
First we need to count the total number of valence cocl2 forms a trigonal planar and has an angle of approximately 120 degrees.
We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. First we need to count the total number of valence cocl2 forms a trigonal planar and has an angle of approximately 120 degrees. Other layer structures include talc and mica (see section 14.9). At room temperature, anhydrous cobalt chloride has the cadmium chloride structure (cdcl 2) (r3m) in which the cobalt under atmospheric pressure, the mass concentration of a saturated solution of cocl 2 in water is about 54. .electrons?what is lewis structure?what is molecular shape?what is bond angle?is cocl^2 polor cocl2 is also called phosgene. The retention of (60)cocl2 (cobaltous chloride) given iv has been studied by total body counting for periods of up to 1000 days. This example problem shows the steps to draw a structure where an atom violates the octet rule. Total # of valence electrons: (ed) sax's dangerous properties of industrial materials. The lewis structure represents the most stable and probable structure for a molecule. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Draw the lewis structure for cocl2, including lone pairs. Thus, the molecular shape of cocl2 is trigonal planar. See the diagram for the lewis structure of cocl2 (phosgene gas). What is the molecular shape of cocl2?
(ed) sax's dangerous properties of industrial materials cdcl2. Clacium carbonate does not have lewis structure as whole compound because it is ion compound.
 Source: images.flatworldknowledge.com
Source: images.flatworldknowledge.com Examples of compounds adopting this structure are fecl2 and c0ci2.
 Source: pubchem.ncbi.nlm.nih.gov
Source: pubchem.ncbi.nlm.nih.gov The individual layers in the two structures are identical, but in cdcl2 the chloride ions are arranged in a ccp lattice, whereas in cdi2 the iodide ions.
 Source: cfile7.uf.tistory.com
Source: cfile7.uf.tistory.com Total # of valence electrons:
 Source: upload.wikimedia.org
Source: upload.wikimedia.org For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
 Source: lookformedical.com
Source: lookformedical.com We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier.
 Source: image.slideserve.com
Source: image.slideserve.com Drawing the lewis structure for cocl2.
 Source: www.sigmaaldrich.com
Source: www.sigmaaldrich.com A simple procedure for writing lewis dot structures was given in a previous post entitled lewis structures and the octet rule.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org How to determine the lewis dot structure of cocl2 and the formal charges of each atom in the molecule.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org Lewis structures and molecular shapes 2.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar.
 Source: image.slideserve.com
Source: image.slideserve.com Let's do the lewis structure for cocl2.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier.
 Source: image.slideserve.com
Source: image.slideserve.com The individual layers in the two structures are identical, but in cdcl2 the chloride ions are arranged in a ccp lattice, whereas in cdi2 the iodide ions.
 Source: cfile7.uf.tistory.com
Source: cfile7.uf.tistory.com The retention of (60)cocl2 (cobaltous chloride) given iv has been studied by total body counting for periods of up to 1000 days.
 Source: image.slideserve.com
Source: image.slideserve.com Home > lewis structures and the octet rule > lewis do structures of phosgene cocl2.
Write bonds in the structure and the place remaining electrons to selected atoms in the structure to give each atom an octet.
 Source: image.slideserve.com
Source: image.slideserve.com Total # of valence electrons:
 Source: upload.wikimedia.org
Source: upload.wikimedia.org A copy of the notes taken.
 Source: media.cheggcdn.com
Source: media.cheggcdn.com Lewis dot structures help predict molecular geometry.
 Source: www.esacademic.com
Source: www.esacademic.com Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org Cadmium chloride forms crystals with rhombohedral symmetry.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar.
 Source: img1.guidechem.com
Source: img1.guidechem.com Write bonds in the structure and the place remaining electrons to selected atoms in the structure to give each atom an octet.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org Let's do the lewis structure for cocl2.
 Source: image2.slideserve.com
Source: image2.slideserve.com We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org How to draw a lewis structure (octet rule exception).
 Source: quizlet.com
Source: quizlet.com Bonds are formed between lone electrons to satisfy the octet rule.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org Other layer structures include talc and mica (see section 14.9).
Posting Komentar untuk "Cdcl2 Lewis Structure"